Batteries in Photovoltaic Systems - Applications and Maintenance
 Apr 11,2025
Apr 11,2025

 sunchees solar system
sunchees solar system
In independent photovoltaic systems, batteries play a very important and critical role as a medium for storing electrical energy. This is because without sunlight, solar photovoltaic systems cannot store electrical energy and deliver it to the load.
When there is no sunlight, we need to store energy in an independent system to run our electrical appliances, and in grid-connected systems, if the power grid is operating normally, then even when there is less or no sunlight, the power grid can provide the required energy.
With technological advancements and market growth, the cost of solar photovoltaic modules is decreasing, while the cost of batteries is becoming a significant component of independent systems. Improper use of batteries can shorten the lifespan of such critical equipment in the system.
A battery is a two-terminal device, with one being the positive terminal and the other the negative terminal. When a battery is charging, a voltage difference is generated across these two terminals. It is precisely because of this voltage difference across the two terminals that current flows when the battery is connected to electrical or electronic devices.
There are various types of batteries available in the market, which differ in shape, size, rated voltage, storage capacity, charge-discharge cycles, battery life, and technology. Batteries are divided into rechargeable and non-rechargeable batteries.
Rechargeable Batteries
Just as adding water to a water tank, independent solar photovoltaic systems also require batteries to restore their charge. The charge level of the battery will decrease as it meets the load demand, just as the water level in a water tank decreases when it is in use.
Once the charge level (like the water level dropping) decreases, it is necessary to continuously replenish the charge to prevent system interruption. This process of restoring charge or recharging the battery is called "charging."
During the charging process, electrical energy is delivered to the battery and is converted into chemical energy there. The process of consuming the battery's electrical energy is called "discharging." During the discharging process, the chemical energy in the battery is converted into electrical energy and delivered to the load.
Therefore, a battery has a charging and discharging process. One complete charging and discharging process of a battery is called a battery charge cycle. Some batteries have multiple charge-discharge cycles, while others do not. Batteries with multiple cycles are called "rechargeable batteries."
These rechargeable batteries with multiple cycles are widely used in solar photovoltaic applications because they ensure continuous power supply to the load even when there is insufficient or no sunlight. Without sunlight, the implementation of independent solar photovoltaic systems would be very unreliable and difficult.
State of Charge (SOC) and Depth of Discharge (DOD)
In reality, not all the electrical energy stored in a battery can be used for load applications. Only a certain proportion of the stored electrical energy can be used to run the load, which is known as the Depth of Discharge (DOD) limit, measured as a percentage.
If the DOD limit of a battery is 30%, then only 30% of the stored electrical energy can be used to run the load. If the DOD limit is 60%, then only 60% of the electrical energy can be used; if the DOD limit is 70%, then only 70% of the electrical energy can be used; if the DOD limit is 80%, then only 80% of the electrical energy can be used to run the load.
Ideally, the DOD limit of a battery should be 100%, and in practical applications, it should be as high as possible. For solar photovoltaic applications, a higher DOD limit percentage is preferred. The DOD limit percentage varies for different batteries; lithium-ion batteries have a DOD limit percentage of about 85% to 90%, while lead-acid batteries, which are most commonly used in solar photovoltaic applications, have a DOD limit percentage of about 50%.
In addition to the DOD limit, DOD can be simply defined as the ratio of the amount of charge used in the battery to the total charge capacity of the battery. The terminal voltage of the battery decreases as the stored charge is used, i.e., DOD increases. The terminal voltage of a fully charged battery is higher than that of a fully or partially discharged battery.
Manufacturers specify the maximum allowable DOD limit for their batteries and recommend not discharging the battery below the specified DOD limit, as this can rapidly shorten the battery's lifespan.
State of Charge (SOC): To determine charging time, it is crucial to understand the current charge level of the battery. The current charge level is represented by the State of Charge (SOC), expressed as a percentage.
For example, an SOC of 70% indicates that the battery stores 70% of its total electrical energy. DOD can also be used to represent SOC, with DOD being the inverse of SOC. If we subtract 70% from 100%, we get 30%, which represents the battery's DOD. It can be mathematically represented by either of the following formulas:
DOD (%) = 100% – SOC (%)
DOD (%) + SOC (%) = 100%
Let's understand this with a simple example: if the DOD is 80%, then by using either of the above formulas, we can determine the percentage of SOC as:
DOD (%) + SOC (%) = 100%
80% + SOC (%) = 100%
SOC (%) = 20%
As we continue to use the battery, the SOC level decreases, while the DOD level increases. It is important to note that the discharge amount of the battery should not fall below the DOD level specified by the manufacturer to avoid shortening the battery's lifespan.
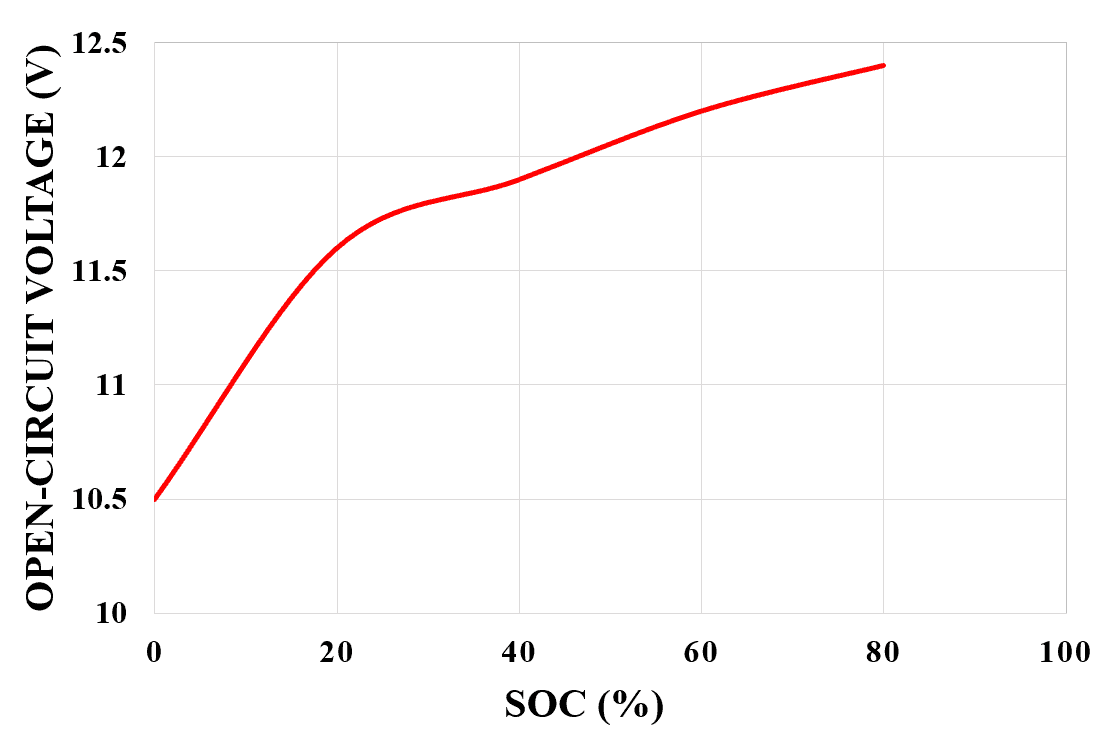
The SOC of a battery can be measured by measuring the terminal voltage of the battery. We know that the SOC of a battery decreases during use, and therefore the terminal voltage of the battery also decreases as the SOC decreases.
Therefore, the higher the SOC, the higher the terminal voltage; the lower the SOC, the lower the terminal voltage. The relationship between the open-circuit voltage of lead-acid batteries and SOC (%) is shown in Figure 3 below.




 Home
Home What is the key role of solar cells in modern solar power generation systems?
What is the key role of solar cells in modern solar power generation systems?  You May Also Like
You May Also Like






 Tel
Tel
 Email
Email
 Address
Address















